Current Projects

Novel mechanism of alcohol self-administration and relapse : R01 AA028782 (Hodge, PI)
Pathological alcohol-seeking behavior is regulated in part by glutamate AMPA receptor (AMPAR) activity in the amygdala. Transmembrane AMPA receptor regulatory proteins (TARPs) profoundly affect the trafficking and function of AMPARs in synaptic and behavioral plasticity. Although the TARP family of proteins is expressed throughout the brain, the TARP γ-8 subtype is restricted to forebrain regions including the basolateral amygdala (BLA); a brain region that is critical to addiction. However, the role of TARP γ-8 in alcohol use disorders (AUD) or other addictions is unknown. To fill this gap in knowledge, we are conducting an innovative set of behavioral, genetic, bidirectional systemic and site-specific pharmacological, molecular, and physiological studies in mice to evaluate the mechanistic role of TARP γ-8 in alcohol reinforcement, escalated self- administration, and cue-induced reinstatement of alcohol-seeking behavior as a model of relapse. Elucidating the neural mechanisms of these three critical behavioral domains has high translational value for understanding the development, progression, and maintenance of AUD. This work moves the field forward in understanding the molecular mechanisms by which alcohol hijacks reward processes and has potential to inform development of new pharmacotherapeutic strategies that target AMPAR function in a highly selective brain region-specific manner.
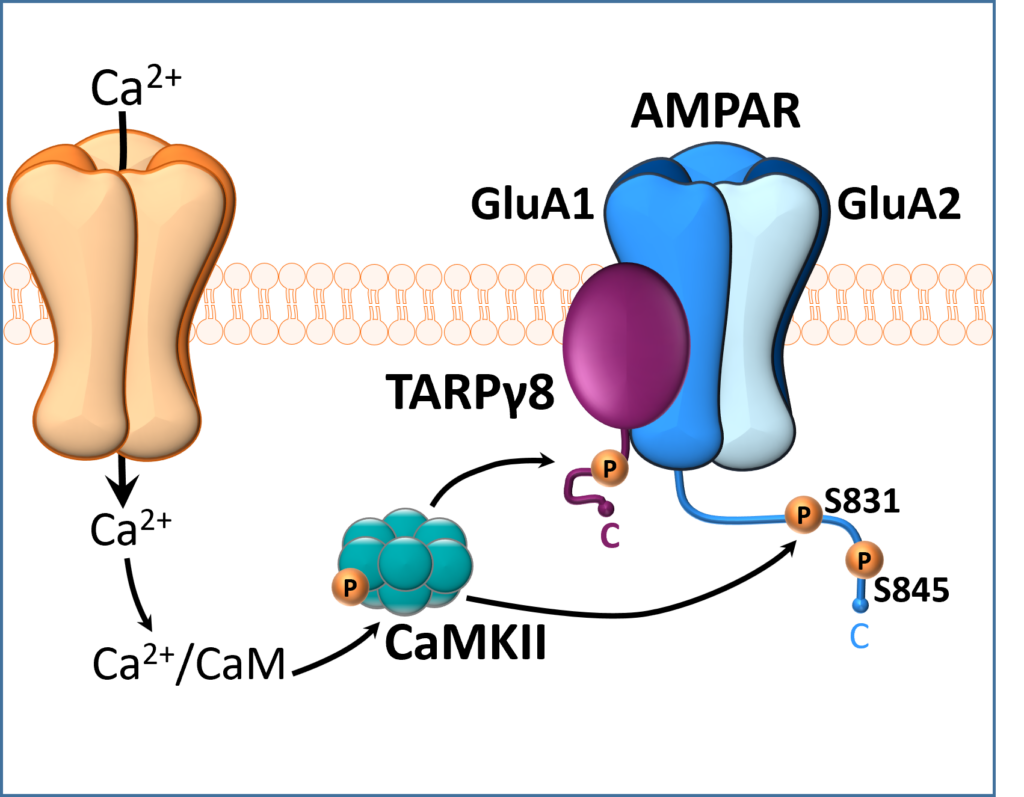
Representation of a glutamate synapse showing CaMKII phosphorylation (e.g., activation) of TARP gamma-8 and the AMPA GluA1 c-terminus. This pathway regulates the reinforcing effects of alcohol.
Impact of alcohol use on tau propagation and neural pathology: R01 AA028782 (Hodge, PI)
Alzheimer’s disease (AD) and its related dementias (AD/ADRD) are a set of irreversible and progressive neurodegenerative disorders that represent the most common cause of dementia in the United States. Tau-associated AD neural pathology originates in the entorhinal cortex (EC) and spreads to the hippocampus (HPC) and other connected brain regions as disease severity progresses. Although links between alcohol use and Tau-associated pathology have been established, the mechanisms that underlie this unique interaction are not fully understood. In this project, we are addressing this gap in knowledge by evaluating the genomic interaction between chronic alcohol drinking and the progression of AD/ADRD neural pathology using an innovative AAV strategy for human Tau-P301L (huTau-P301L) “seeding” in the EC of C57BL/6J mice. We are using GeoMx Digital Spatial Profiling (DSP) with quantification by next-generation sequencing (NGS) to evaluate the interaction between alcohol drinking and huTau-P301L expression on the whole transcriptome (>22,000 genes) from neurons and glia in the EC, hippocampus, and other connected brain regions. We will also evaluate pathological huTau-P301 expression in the EC, propagation (spread) to the HPC and other nuclei, Tau misfolding, and Tau (AT8) hyperphosphorylation in a parallel immunohistochemistry study. This work is based on strong initial data showing that alcohol drinking produced an early onset and profound increase in pathological human Tau-AT8 phosphorylation in the hippocampus that were time-locked with early onset and increased magnitude of hippocampal-dependent cognitive deficits in 3xTg-AD mice. Given this prominent involvement of Tau in our prior alcohol studies and its widely acknowledged function as a key mechanism of AD/ADRD, we predict that these studies have a high probability of identifying a unique transcriptomic footprint of alcohol that contributes to AD/ADRD pathology. Identifying novel neural targets of alcohol that underlie AD/ADRD pathology has potential to lead to specific diagnostic and therapeutic strategies.
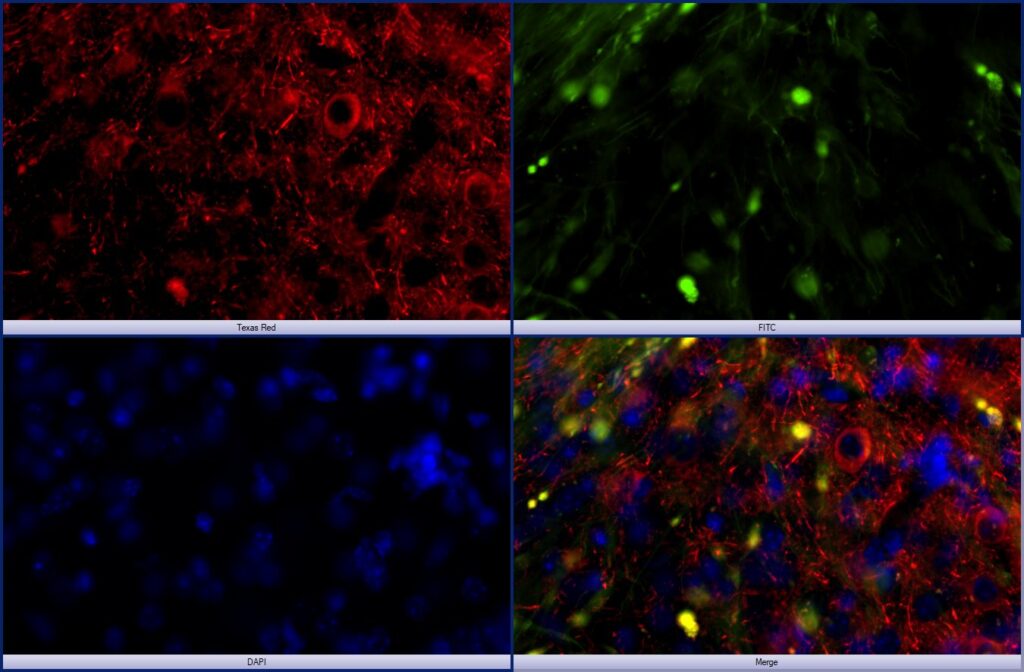
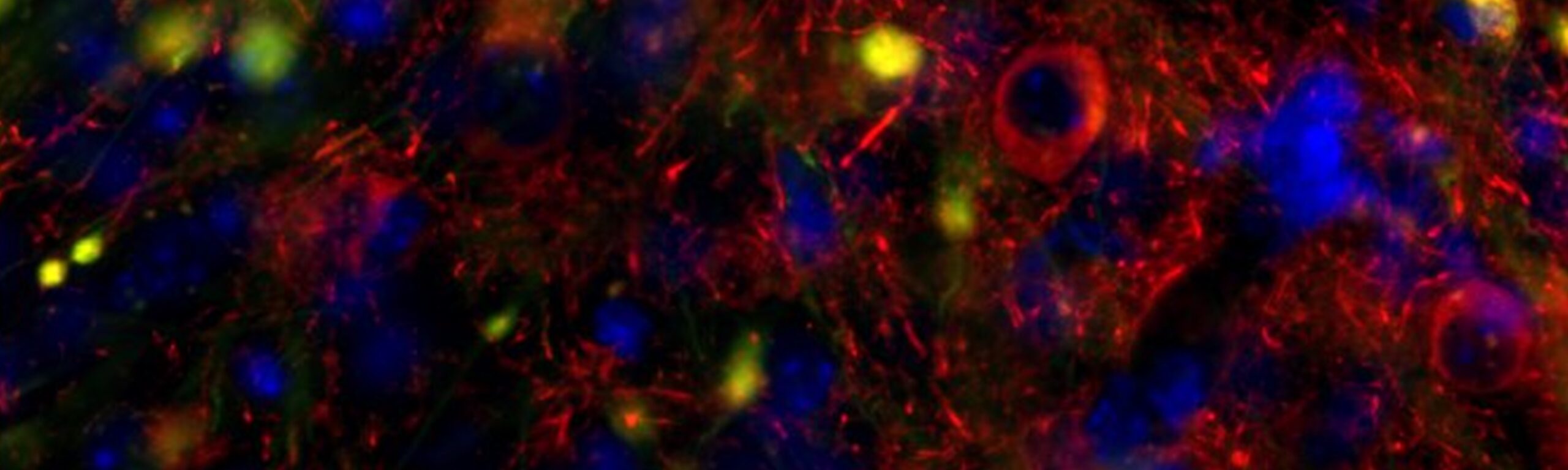
Image showing Human Tau expression in mouse amygdala after tau seeding in the entorhinal cortex. Red=human tau; green=GFP; blue=DAPI.
Limbic glutamatergic circuits in ethanol dependence and escalated operant self-administration: P60AA011605 (Hodge, PI; McElligott, co-PI)
Initial discoveries in our component of the UNC Center Grant show that alcohol dependence both escalates the reinforcing effects of alcohol, as measured by operant self-administration, and upregulates AMPAR expression in the basolateral amygdala (BLA) and other brain regions that send glutamatergic projections to the nucleus accumbens (Acb), a central component of the reward pathway. This convergence of molecular and behavioral data supports the overall hypothesis that: AMPAR expression and activity in the BLA–>Acb pathway is a target of alcohol dependence that, in turn, drives escalated alcohol self- administration. We are testing this hypothesis at the molecular, physiological and behavioral levels, which include using an innovative CRISPR/Cas9 method for specifically deleting the GluA1 subunit in this neural circuit. Multi-channel fiber photometry will be used to assess the impact of alcohol dependence on calcium signaling in genetically tagged excitatory and inhibitory cells during escalated operant alcohol self-administration. These groundbreaking studies will move the field forward in understanding how calcium-dependent glutamatergic mechanisms in the BLA–>Acb circuit regulate the escalated reinforcing effects of alcohol associated with AUD.
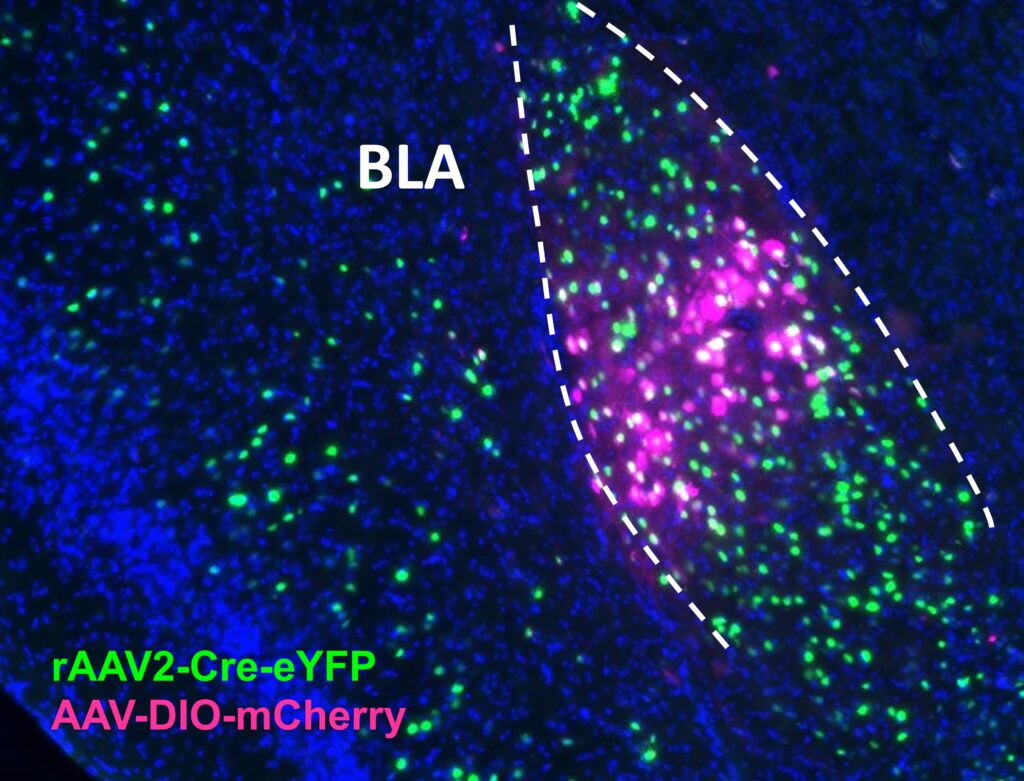
Expression in BLA of retrograde AAV2-Cre-eYFP injected in Acb and AAV-DIO-mCherry injected in BLA. From The McElligott Lab.
Pathological AMPA receptor adaptations governing dependence escalated alcohol self-administration: K99AA029754 (Hoffman, PI; Hodge, Sponsor)
Alcohol dependence and multiple withdrawal experiences are related to increased severity of alcohol use disorder (AUD), craving, and resistance to treatment. Alcohol abuse gains control over behavior, in part, through pathological adaptations of glutamatergic AMPA receptor (AMPAR) mechanisms that regulate synaptic and behavioral plasticity in brain reward pathways. The unique auxiliary protein, transmembrane AMPAR regulatory protein (TARP) γ-8, has been shown to regulate AMPAR trafficking, activity, and CaMKII-dependent plasticity, making it critical for AMPAR mediated neural transmission. Since AMPAR activity is required for the development of new behavior (e.g., learning) and retention of actions (e.g., memory), this fundamental neural process may underlie the development, maintenance, and critically, dependence-escalated self-administration of alcohol. Therefore, this K99/R00 proposal will determine if TARP γ-8 regulates AMPAR mediated transmission in key brain regions during dependence-escalated alcohol self-administration. Aim 1 (K99 phase) of the proposal will investigate the role of TARP γ-8 dependent excitatory Ca2+ signaling in reward-related brain regions during alcohol self- administration in the mPFC, BLA, NAc, and vHPC using a highly novel multi-spectral, four-channel fiber photometry platform. Aim 2 (K99 phase) will examine TARP γ-8 as a mechanism of CIE vapor dependence- induced escalation of alcohol self-administration and the consequential co-localization of TARP γ-8 and AMPAR using confocal microscopy. Aim 3 (R00 phase) combines these techniques to evaluate Ca2+ signaling in key- reward brain regions during dependence-escalated alcohol self-administration. These findings are then extended by taking a circuit-based approach using a selective pharmacological manipulation in combination with fiber- photometry to evaluate site-specific TARP γ-8 bound AMPAR inhibition on “bottom-up” (BLA to NAc) and “top- down” (mPFC to NAc) Ca2+ signaling. This work moves the field forward by providing fundamental mechanistic insights into TARP γ-8 dependence-escalated alcohol self-administration which has high translational value for understanding and treating AUD and has the potential to inform development of new pharmacotherapeutic strategies that target AMPAR function in a highly-selective brain region specific manner.
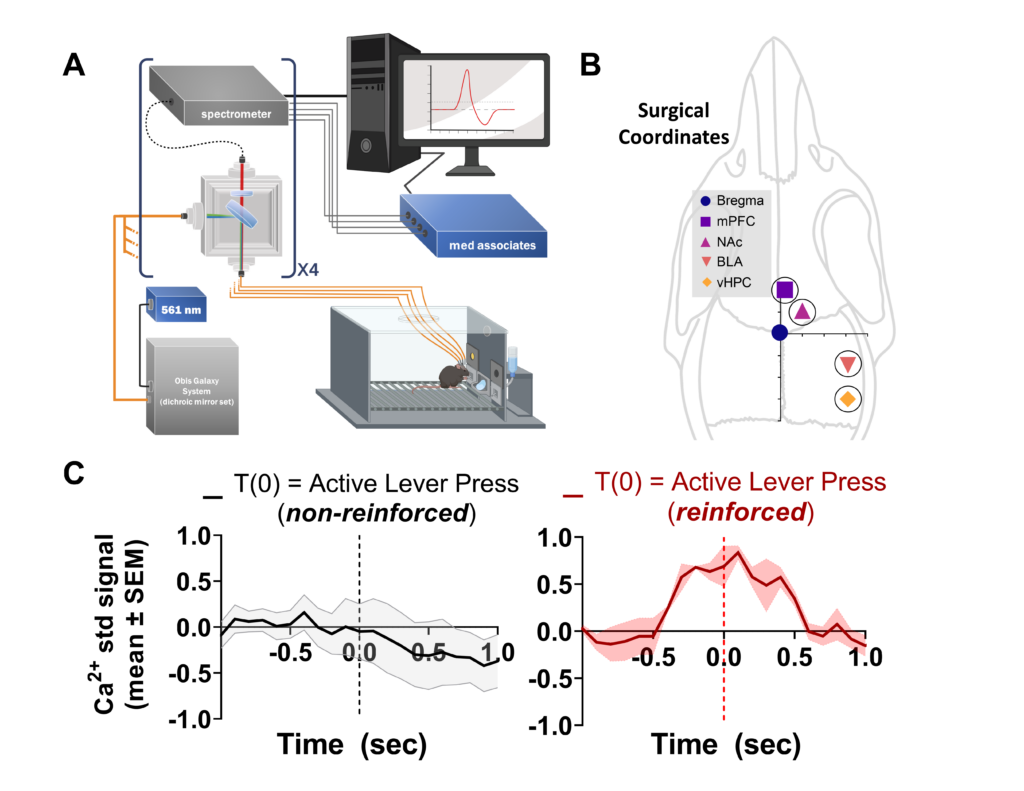
Fiber photometry system and results showing calcium signal recorded from mouse neurons in the amygdala during operant alcohol self-administration.

A novel glutamatergic mechanism for treatment of cocaine and opioid use disorders, and co-occurring anxiety (Faccidomo, PI)
Need/Problem: Despite tremendous need, effective pharmacotherapies for the treatment of Substance Use Disorders and co-occurring psychiatric conditions are lacking. Repeated drug use dysregulates key neurotransmitter systems in the brain and leads to enduring changes in strength and function of brain pathways that regulate reward and pleasure. It has been a historical challenge for drug development to selectively target these pathways to address co-occurring mental health conditions without undesirable side effects. This proposal offers and innovative and selective approach to targeting glutamate that may mitigate some of these side effects. Project Summary: Drugs like cocaine and morphine increase the strength of excitatory projections in the brain which can lead to long lasting changes in brain activity and function. I will use preclinical mouse models of cocaine and opioid use disorders, to address whether a glutamatergic protein called TARP γ-8 is a common neural mechanism of drug reward and anxiety. Goals & Projected Outcomes: The goal of this project is to determine whether TARP γ-8 regulates the rewarding properties of cocaine and morphine. I predict that blunted drug reward and anxiety-like behavior will be seen in 1) mice lacking the gene for TARP γ-8 and 2) after administration of a novel therapeutic drug, JNJ-5, that selectively inhibits TARP γ-8. Grant Details: I believe that selective pharmacological interventions could be successful in mitigating some of the adverse side effects of therapeutic drugs that interfere with compliance. The auxiliary glutamate receptor protein, TARP γ-8, is an ideal candidate for targeted pharmacotherapy because it has very discrete anatomical specificity in the brain. In this grant, I will use a genetic and a pharmacological approach, to ask whether TARP γ-8 is a critical mechanism that regulates cocaine and morphine reward, learning and associated anxiety. First, I will use a mouse model that lack the gene for TARP γ-8 and will assess whether these mice show blunted neuroadaptations, reward and anxiety to repeated cocaine and/or morphine administration. Second, I will test whether the drug JNJ-5, a selective inhibitor of TARP γ-8, is effective at blocking cocaine and morphine neuroadaptations, reward and anxiety. These preclinical studies will establish whether TARP γ-8 is a novel glutamatergic target for selectively blunting the rewarding properties of drugs of abuse and anxiety without disrupting other, pleasurable activities.
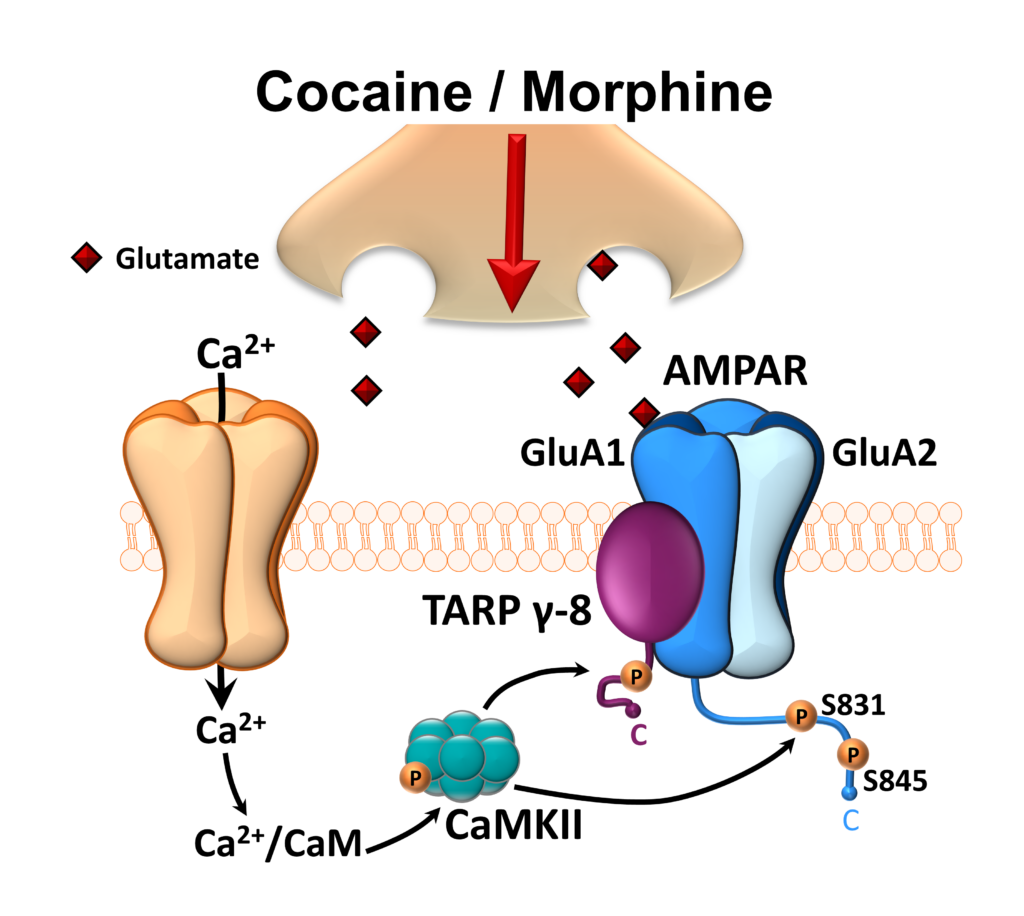
Glutamate synapse showing TARP gamma-8 as the proposed site of action where cocaine and morphine act to enhance drug reward and anxiety. To test this hypothesis, TARP gamma-8 will be manipulated using gene knockout mice and a selective pharmacological inhibitor.